It’s not a shock. It’s hardly even news. I’ve been pointing out for a long time that Bay Area Lyme Foundation has some rather unsavory characters on its scientific advisory panel. But the desire among Lymish for BALF to be on the side of *good* is strong enough to induce a willing suspension of disbelief. My constantly pointing out that they work with Alan Barbour (for God’s sake) falls on ears that are deaf to a reality that pits one of the most celebrated Lyme “nonprofits” directly against the people they claim to be helping.
Shall we change that? I’m going to bring you along on a typical research session, where I begin with a simple question and end up down the darkest of bunny trails.

I started on PubMed wondering, “anything new about borrelia?” Adriana Marques, Chief, Lyme Disease Studies Unit, Laboratory of Clinical Immunology and Microbiology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, had something new.
Adriana Marques, who in 2006 reported that Lyme causes humoral immunosuppression with chronic brain inflammation (https://www.ncbi.nlm.nih.gov/pubmed/16783164/) has just endorsed a “modified two tier test” for Lyme in this report: https://www.ncbi.nlm.nih.gov/pubmed/29898997/
This is the test she specifically refers to:
http://jcm.asm.org/content/early/2018/05/03/JCM.01943-17.abstract
This is a test method for early detection of late Lyme arthritis. It is even worse than what we have now because doctors will be emboldened to dismiss symptoms immediately. Note the use of “well characterized serum samples” from the CDC repository. That means they are only looking at the approximately 15% of Lyme borreliosis cases who have an HLA-linked arthritic knee—not the cases who walk the line between life and death every single day.
“The purpose of this study was to evaluate the performance of the VlsE/C6 MTTT using well-characterized serum samples. Serum samples from the CDC Lyme Serum Repository were tested using three MTTTs: VlsE/C6, whole cell sonicate (WCS)/C6 and WCS/VlsE, and three STTTs (immunoblots preceded by three different first-tier assays: VlsE, C6 and WCS).”
These are the authors and their affiliations:
Adoracion Pegalajar-Jurado(a), Martin E. Schriefer(a), Ryan J. Welch(b), Marc R. Couturier(b,c), Tiffany MacKenzie(d), Rebecca J. Clark(a), Laura V. Ashton(a), Mark J. Delorey(a) and Claudia R. Molins(a)
a) Division of Vector-Borne Diseases, Centers for Disease Control and Prevention, Fort Collins, Colorado, USA
b) ARUP Laboratories, Salt Lake City, Utah, USA
c) Department of Pathology, University of Utah, Salt Lake City, Utah, USA
d) DiaSorin Inc., Stillwater, Minnesota, USA
DiaSorin?
$$$$$$$$$$$$$$$$$$$$$$
Gary Wormser consults for DiaSorin.
See a couple of his his conflict of interest statements: https://idsa.confex.com/idsa/2009/webprogram/Paper28553.html
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4489928/
The same Gary Wormser who has three times published that OspA causes immunosuppression.
The same Gary Wormser who is currently being sued for RICO and antitrust.
The same Gary Wormser who was on the IDSA Lyme guidelines committee.
The same Gary Wormser who was a founder of ALDF, the central racketeering organization of the whole Lyme nightmare that never ends.
😒
$$$$$$$$$$$$$$$$$$
Meanwhile, in Boston…
Allen Steere and Lise Nigrovic (and others) ALSO endorsed “modified two tier testing” in this report….
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5399943/
funded by….
“This work was supported by research grants from DiaSorin, Inc, and the Bay Area Lyme Foundation to J. A. B.”
Diasorin and who? Right. Bay Area Lyme Foundation.
$$$$$$$$$$$$$$$$$$$$$$$$$$$$$$
J.A.B., in case you don’t know, is John A. Branda, who works with Steere at Mass General:
https://www.massgeneral.org/doctors/doctor.aspx?id=17863
How clever of them to pass all the money through Branda, the relative nobody, to avoid the Lyme Luminaries, Steere and Nigrovic, having to report it in the future as a conflict.
Here are the conflicts of interest from the Steere/Nigrovic/Branda Report, by the way:
Potential conflicts of interest. J. A. B. has received research support from Immunetics, Alere, bioMérieux, and the National Institute of Allergy and Infectious Diseases (NIAID; award number 1R21AI119457-01) for other research projects, and has served as consultant to T2 Biosystems. A. C. S. has received research support from the NIAID (award number R01A1101175), the English-Bonter-Mitchell Foundation, and the Eshe Fund. K. S. has received research support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases at the National Institutes of Health (NIH; award number K01AR062098). P. M. L. has received research support from the National Center for Advancing Translational Sciences of the NIH (award number KL2 TR001115). L. E. N. has received research support from the Harvard Catalyst, the Boston Children’s Hospital Faculty Council, the Bay Area Lyme Foundation, and the Centers for Disease Control and Prevention. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Oh, also:
“The authors thank Claudia R. Molins and Martin E. Schriefer for their input and support.” (Recall that these two are CDC authors of the DiaSorin test report above—the one reviewed by Adriana Marques—which was coauthored by DiaSorin employees.)
You might remember that this Molins woman went on TV last year to lie to patients about developing more accurate tests. Here she is in a Scientific American article:
“Lyme disease is a serious problem here in the United States and we really need to find solutions to some of the limitations that we have, particularly in diagnosing this infection,” said Claudia Molins, a microbiologist at the CDC. “We want a test that can be used within the first two weeks of infection and that does not rely on antibody production.”
The article continued:
“They are also working on developing better biosignatures and algorithms for identifying Lyme based on new blood samples they recently collected from clinics affiliated with Colorado State University and New York Medical College. Eventually they will begin real-time testing on patients’ serum samples collected in clinics and mailed to the CDC.” (Those would be the fabled “well characterized” samples.)
https://www.statnews.com/2017/06/28/early-lyme-tests/
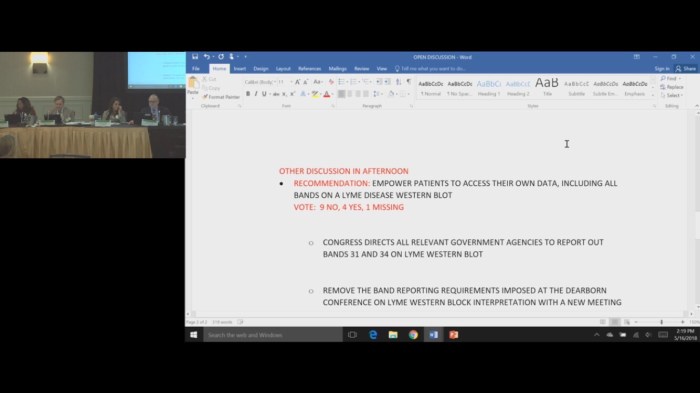
It’s no wonder the HHS tick borne disease working group voted down the proposal to allow patients to see the results of ALL of their Western blot bands. The crooks are doing away with the Western blot altogether. I guess they figure that since we have one foot in the grave, they can go ahead and start shoveling in the dirt.
Categories: Lyme Disease

 Arthritic Knee Syphilis
Arthritic Knee Syphilis  Wonkless: A Satirical Christmas Tale of Lyme & Consequences
Wonkless: A Satirical Christmas Tale of Lyme & Consequences  An Institutional Form of Murder
An Institutional Form of Murder  Why There Can’t be a Real Lyme Test
Why There Can’t be a Real Lyme Test
I thought it was interesting that someone who worked at a foundation which is known for allocating questionable “dark money” took a job for the Bay Area Lyme Foundation.
The Philanthropy Roundtable is outspoken in its opposition to transparency. They are known for keeping donors identities confidential. Ana Thompson was on the Board of Directors of the Roundtable. She left the roundtable and took a job as Executive Director of the Bay Area Lyme Foundation and held that job from May 2012 to May 2014.
https://www.sourcewatch.org/index.php/Philanthropy_Roundtable
https://www.linkedin.com/in/ana-thompson-7aa2a11
LikeLiked by 1 person